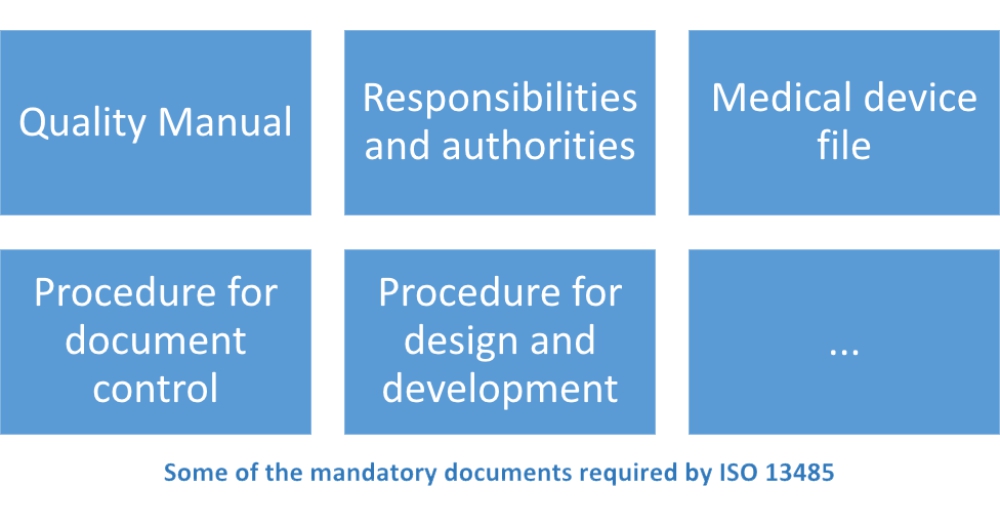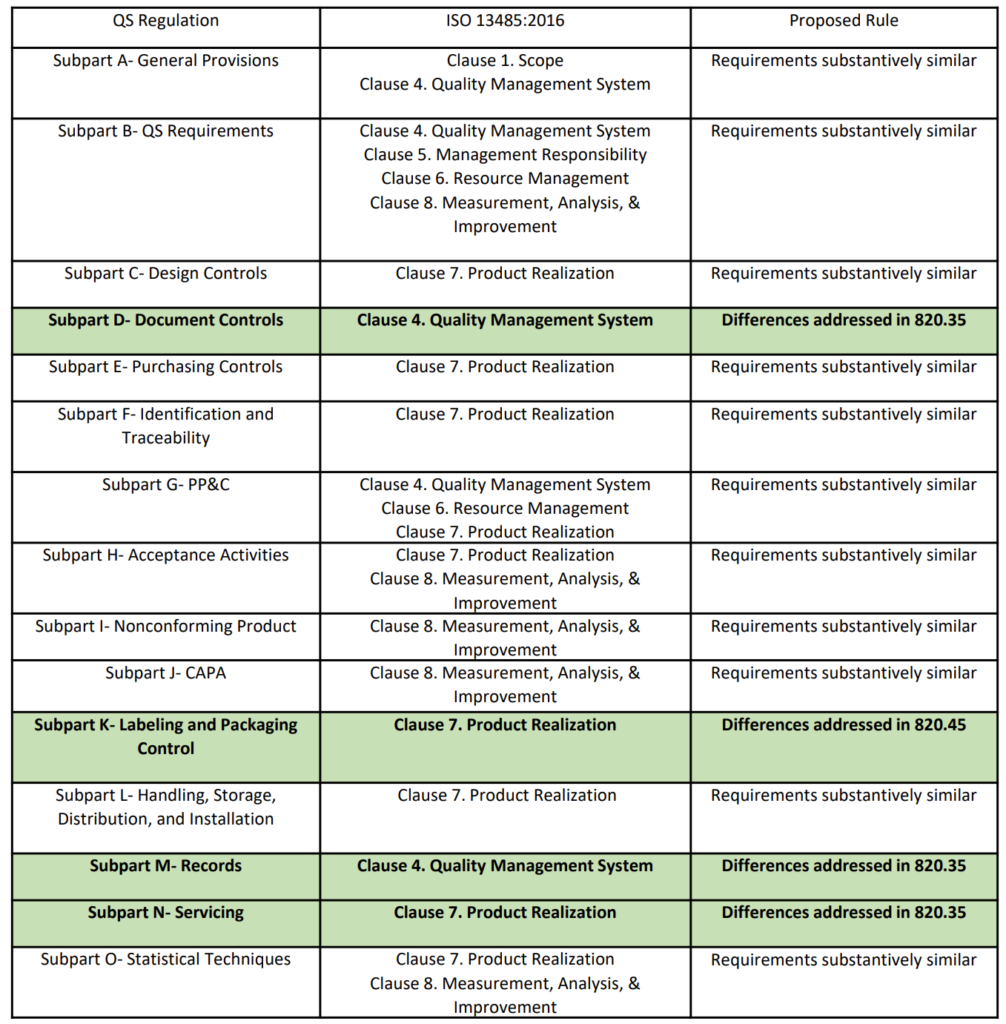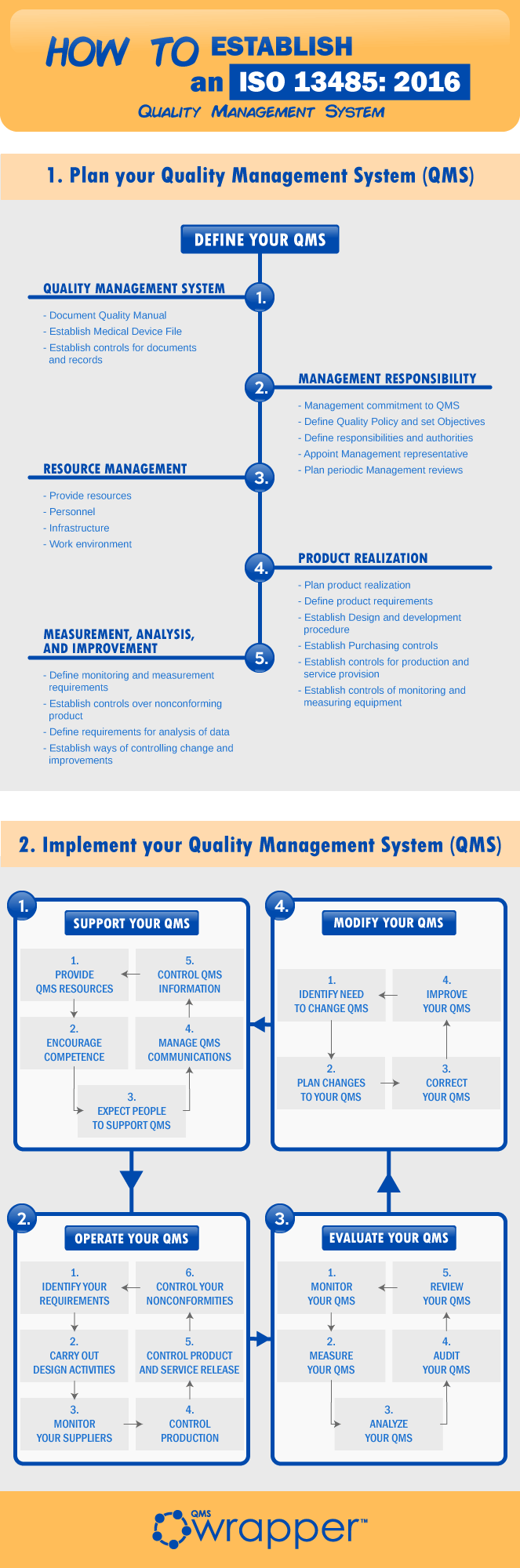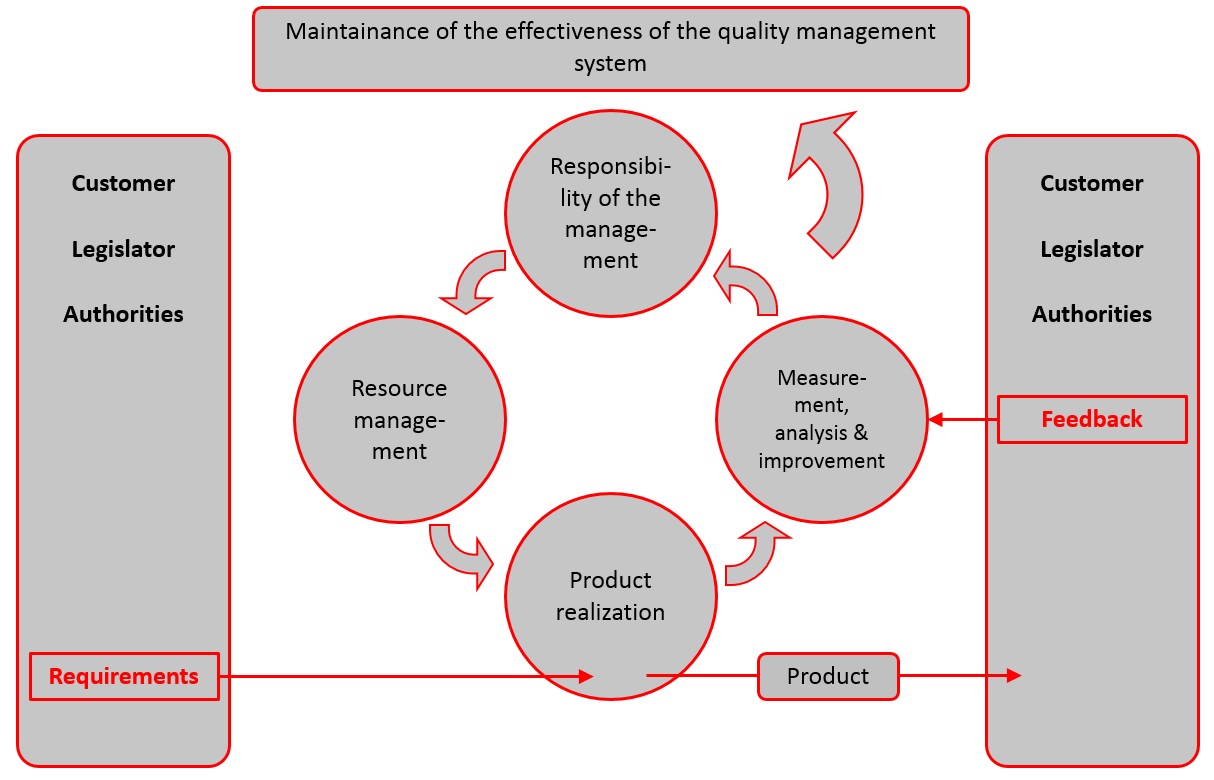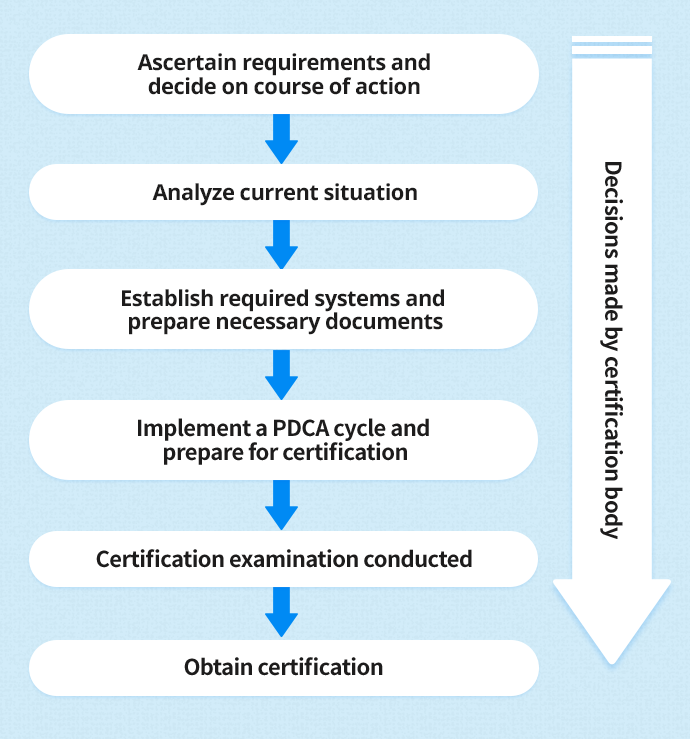
Acquisition of ISO 13485 certification - Total support for medical devices regulatory affairs / SunFlare Japanese

ISO 13485:2016 - Medical devices — Quality management systems — Requirements for regulatory purposes

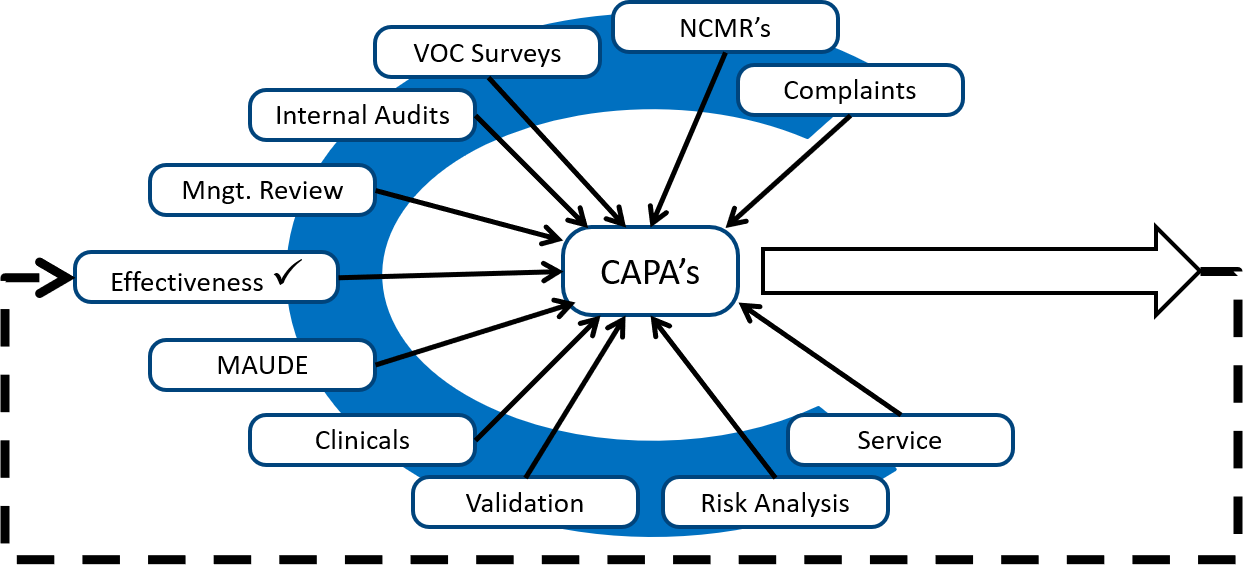
MD-QMS Measurement, Analysis and Improvement Clause 8 of ISO 13485:2016| Training on ISO 13485:2016| - YouTube

MD-QMS Measurement, Analysis and Improvement Clause 8 of ISO 13485:2016| Training on ISO 13485:2016| - YouTube

MD-QMS Measurement, Analysis and Improvement Clause 8 of ISO 13485:2016| Training on ISO 13485:2016| - YouTube
ISO 13485:2016 - Medical devices — Quality management systems — Requirements for regulatory purposes


.gif)