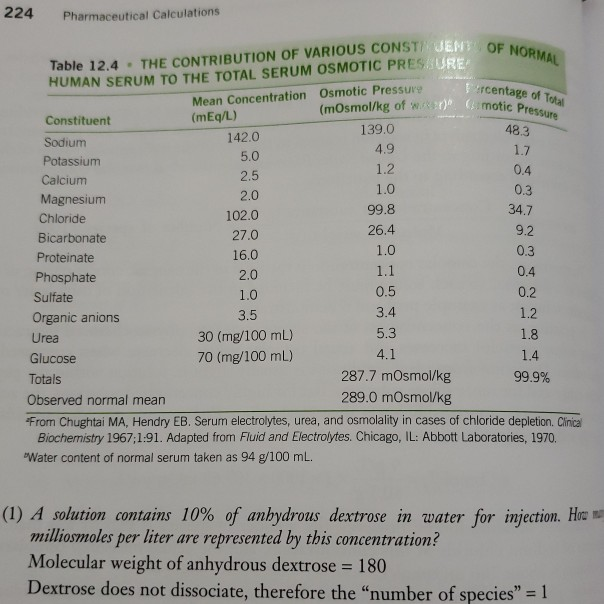
ph.jawzaa|ص.جوزاء on Twitter: "Tomorrow will continue with powders reconstitution calculations 💉💊 and enjoy the injection preparation video 🤩👏🏻 https://t.co/8bJAt9vYjB" / Twitter
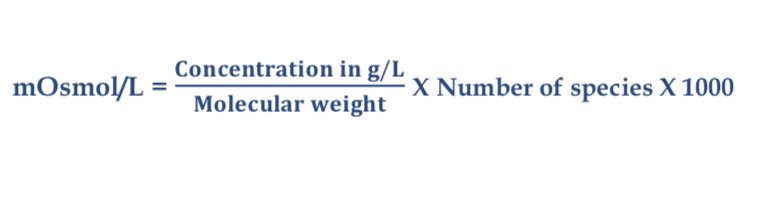
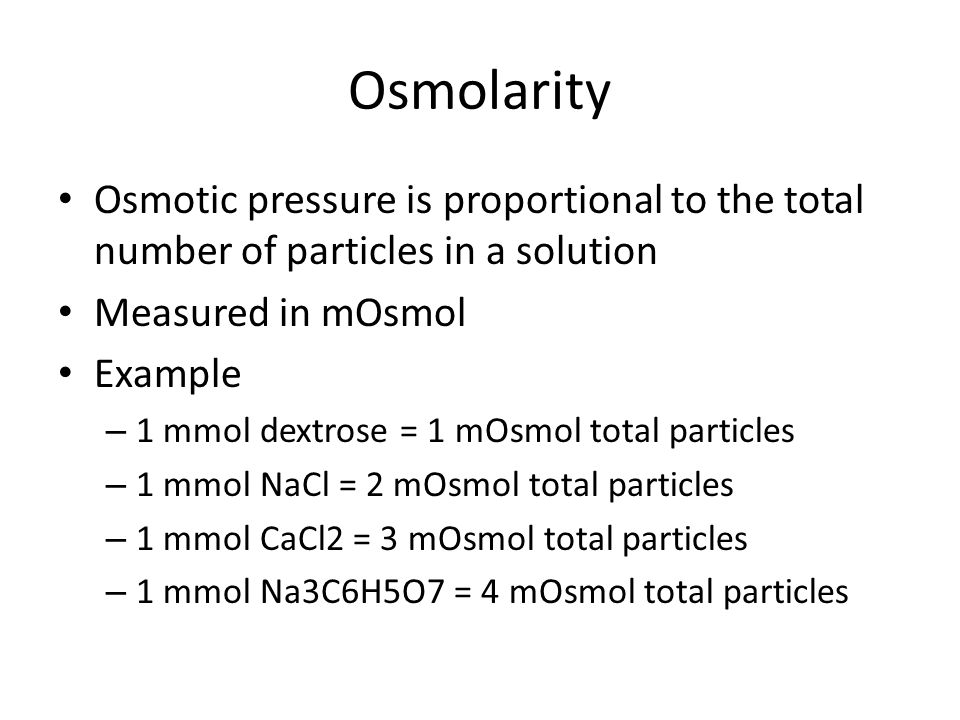
ph.jawzaa|ص.جوزاء on Twitter: "Example 1: Calculate the ideal osmolarity of 0.9 % NaCl injection? Because of bonding forces, however, n is slightly less than 2 for solutions of sodium chloride at this
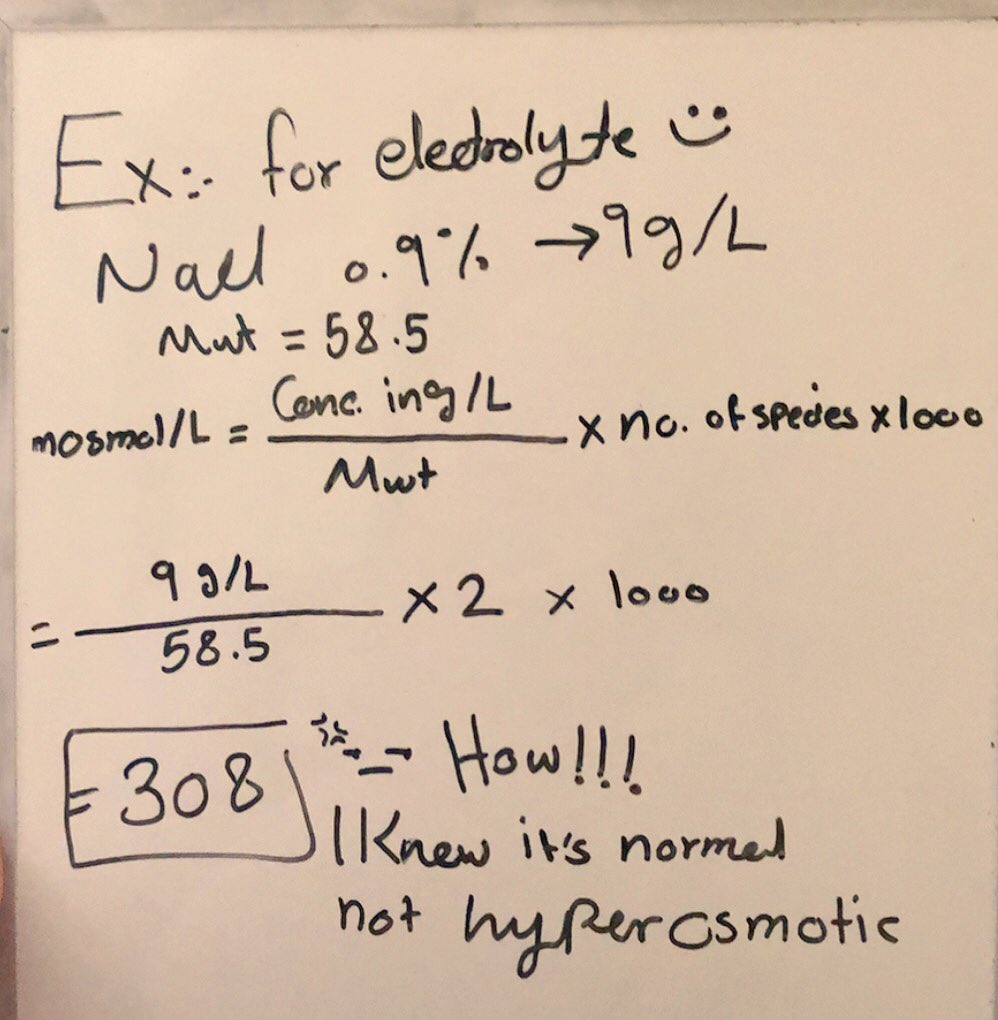
ph.jawzaa|ص.جوزاء on Twitter: "Example 1: Calculate the ideal osmolarity of 0.9 % NaCl injection? Because of bonding forces, however, n is slightly less than 2 for solutions of sodium chloride at this
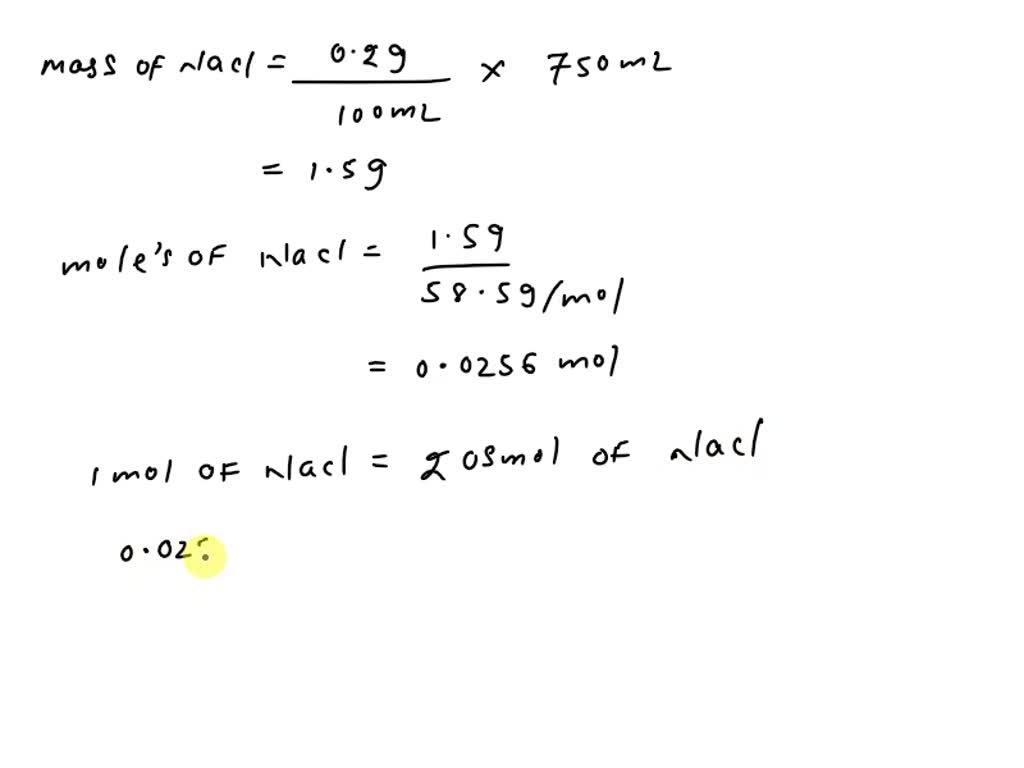
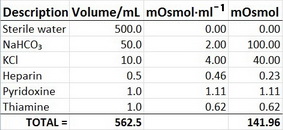
SOLVED: What is the Osmolarity(mOsmol/L) of a 750 mL parenteral solution containing 2 % Dextrose (MW = 180) and 0.2 % Sodium Chloride ( MW = 58.5). Ammonium Chloride injection contains 250
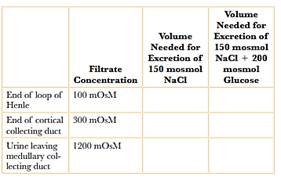
Solved) - Osmotic diuresis refers to the loss of additional water in urine... (1 Answer) | Transtutors
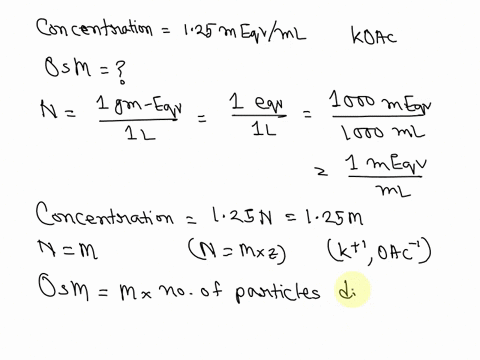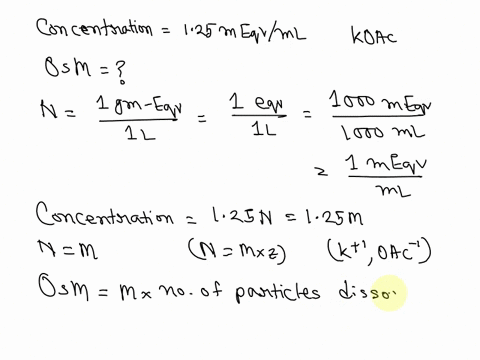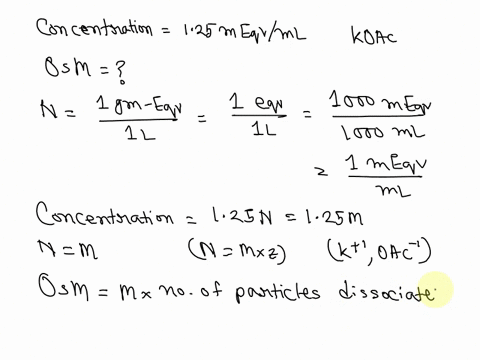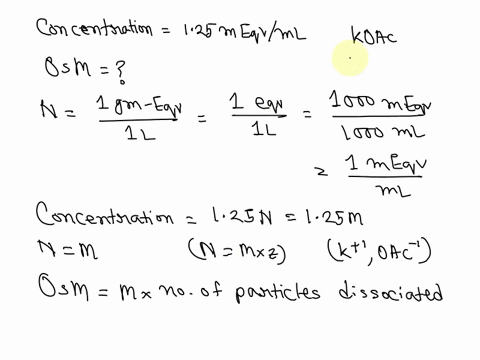
SOLVED: What is the Osmolarity(mOsmol/L) of a 750 mL parenteral solution containing 2 % Dextrose (MW = 180) and 0.2 % Sodium Chloride ( MW = 58.5). Ammonium Chloride injection contains 250