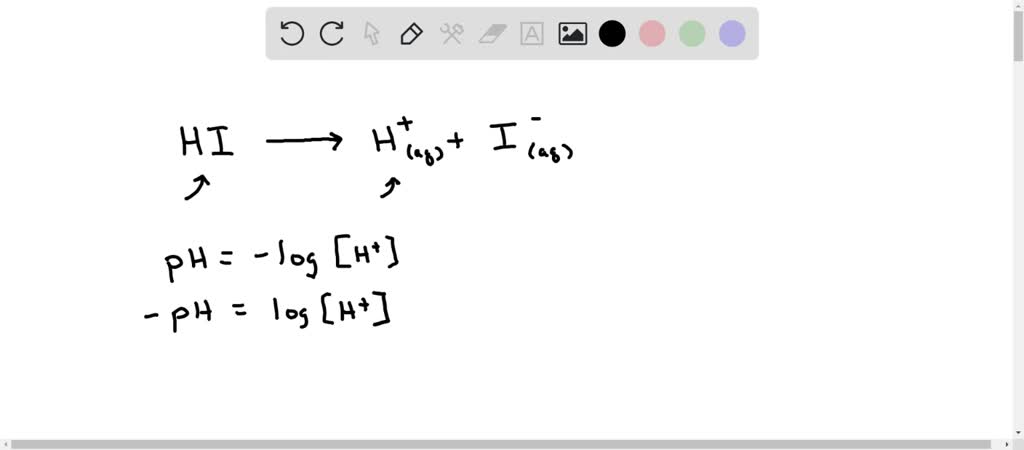
SOLVED: Calculate the concentration of an aqueous HI solution that has pH=2.50 . HI is a strong acid.

Calculating Hydronium ion concentration from pH (and identifying molecules based on pH) : r/chemhelp
![SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice, SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,](https://cdn.numerade.com/previews/c92ef7f1-c83f-4027-9f05-6b11ebe1b21e_large.jpg)
SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora
![Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download](https://images.slideplayer.com/17/5302527/slides/slide_3.jpg)




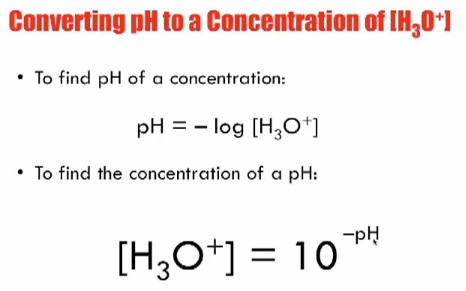
:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)


![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)

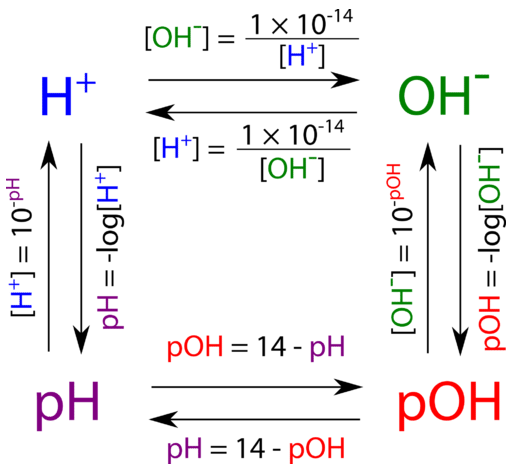
![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)




![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)