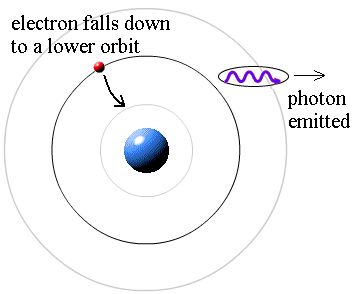
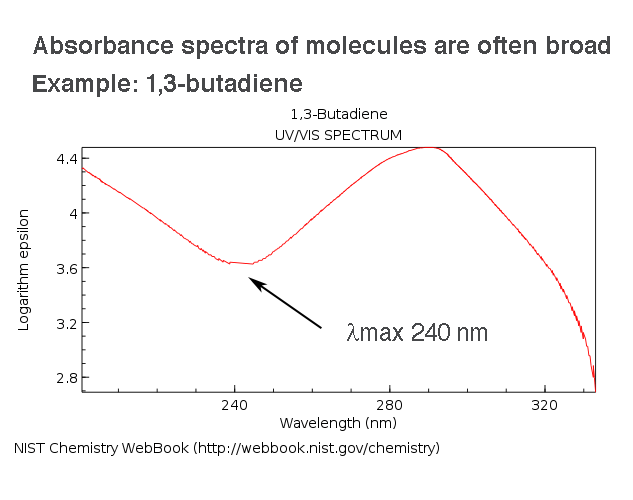
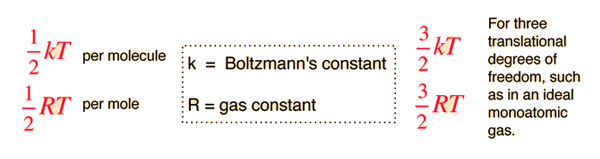I have a question where I have to find out the maximum kinetic energy from the wavelength, frequency of photon and stopping potential. How do I find the maximum kinetic energy from

PDF) Shape and size of electron are determine by the amount of photon energy incidence on it 5e7f7e40d7564 (1) | Saddam H U S A I N Dhobi - Academia.edu

Electron–vibration coupling induced renormalization in the photoemission spectrum of diamondoids | Nature Communications

About these slides These slides are used as part of my lessons and shouldn't be considered comprehensive There's no excuse for not turning up to lessons! - ppt video online download

Coupling between Harmonic Vibrations Influences Quantum Beating Signatures in Two-Dimensional Electronic Spectra | The Journal of Physical Chemistry C

Question Video: Calculating the Kinetic Energy of an Electron Moving through a Negative Potential Difference | Nagwa

Vibration-to-electricity dynamic conversion scheme. Energy balance: the... | Download Scientific Diagram