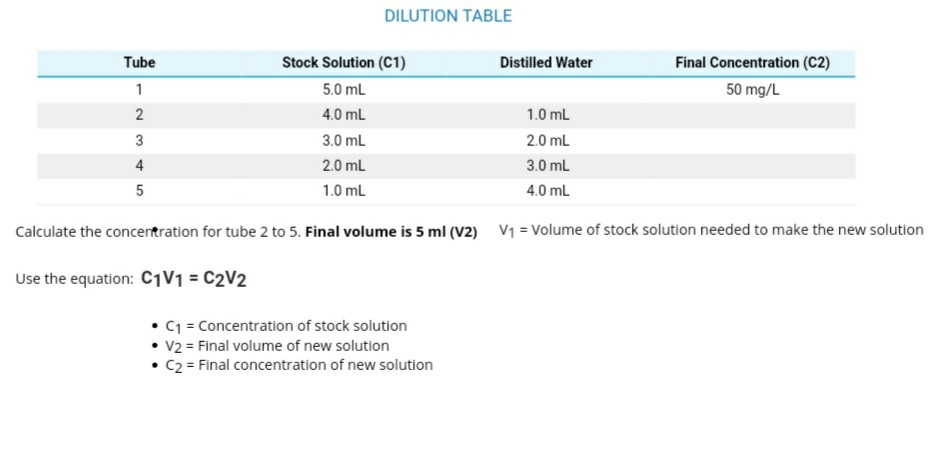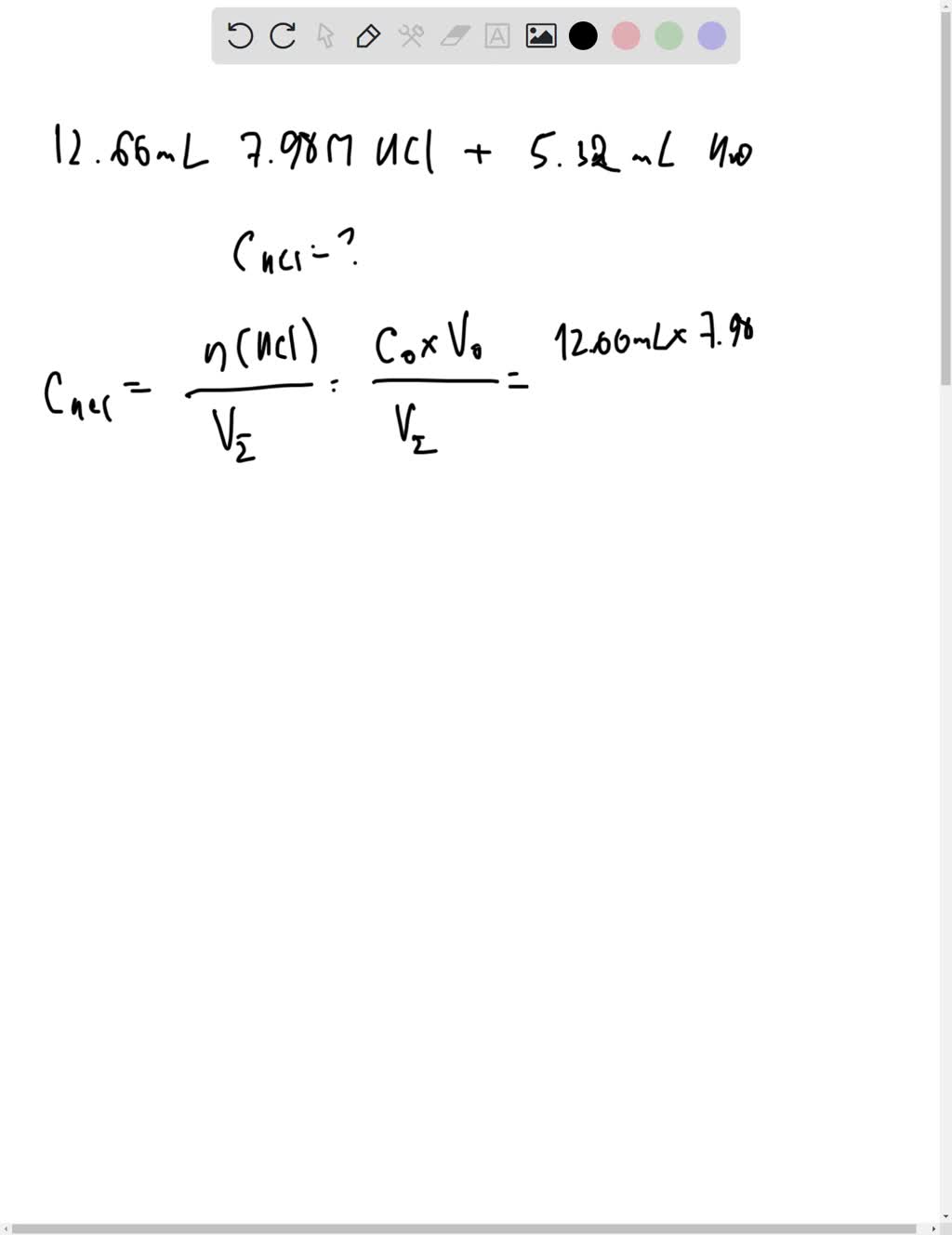
SOLVED: You will be performing many dilutions in this experiment. If John adds 12.66 mL of 7.98 M HCl to 5.32 mL of distilled water, what is the final concentration, in M, of HCl?

Concentration of magnesium (mg L−1) in distilled water and artificial... | Download Scientific Diagram

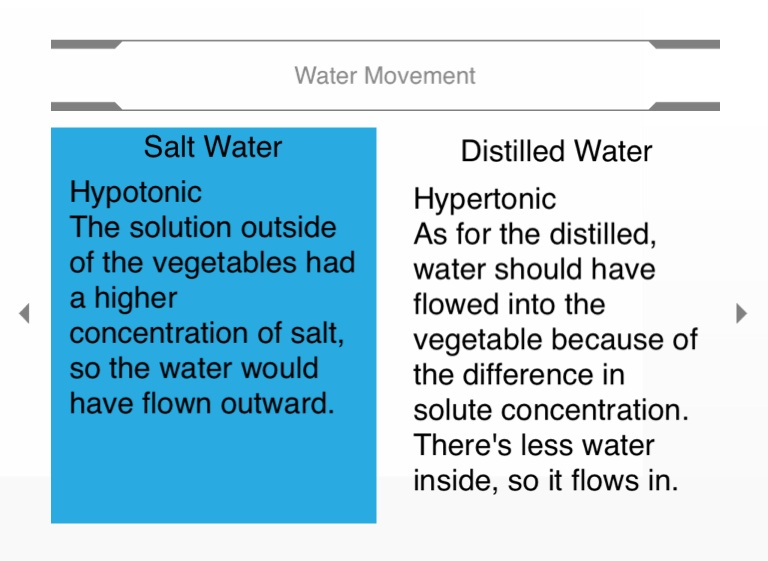
Water moved into the egg in distilled water because the higher concentration of water was outside the cell so it diffused inside t… | Glassware, Osmosis, Corn syrup
![At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube](https://i.ytimg.com/vi/sG9XjpQwtSA/maxresdefault.jpg)
At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube

pHgraduatedscale.jpg - Concentration of Hydrogen ions compared to distilled water Examples 10 000 000 PH 0 Battery acid 1 000 000 PH 1 Hydrochloric | Course Hero

You will be performing many dilutions in this experiment. If John adds 17.51 mL of 6M HCl to 6.36 mL of - Brainly.com

Alexis placed one egg in a solution of 95% corn syrup. She placed another egg in distilled water. Both eggs - Brainly.com






:max_bytes(150000):strip_icc()/water_annotated-4ab4020558324528a40132c0d842754b.jpg)