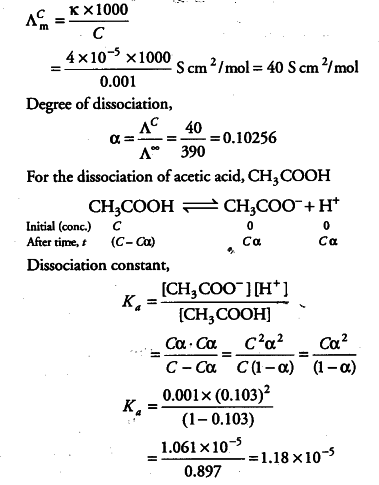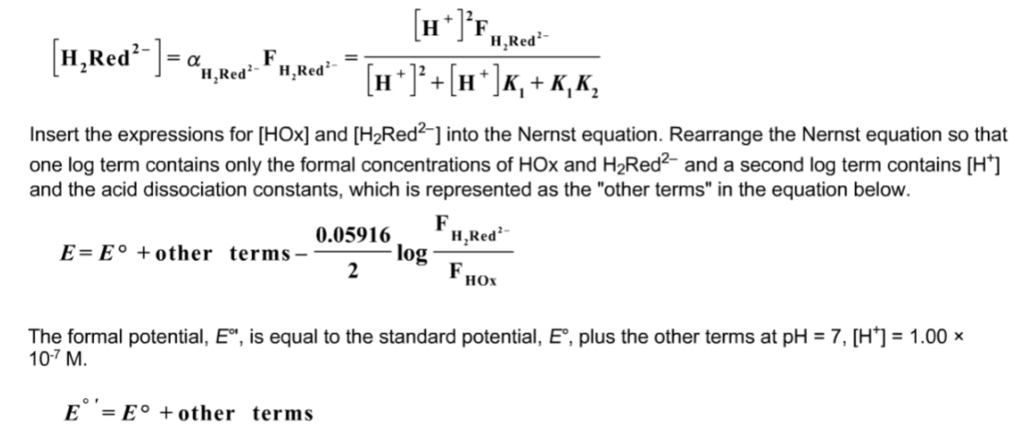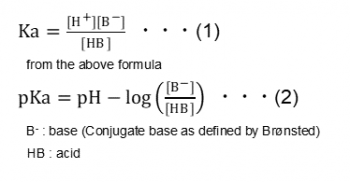
How should the acid dissociation constant pKa be measured? | Automatic Potentiometric Titrators | Faq | Kyoto Electronics Manufacturing Co.,Ltd.("KEM")
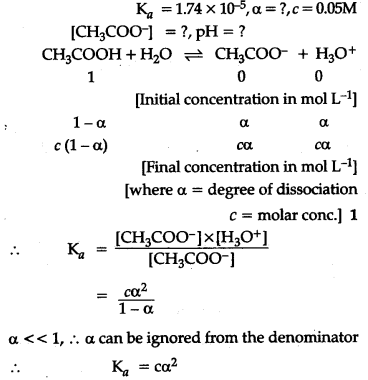
The ionization constant of acetic add is 1.74 x${{10}^{-5}}$ . Calculate the degree of dissociation of acetic add in its 0.05 M solution. Calculate the concentration of acetate in the solution and

Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

Autoionization & Dissociation Constant of Water | Autoionization & Dissociation of Water Equation & Examples - Video & Lesson Transcript | Study.com

Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

the dissociation constant of a weak acid h a is 1 2 into 10 to the power minus 10 calculate - Chemistry - Equilibrium - 13209509 | Meritnation.com

equilibrium - How to calculate the dissociation constant of a weak acid from the titration with a strong base? - Chemistry Stack Exchange
Determination of the dissociation constant (K d ) of the full-length... | Download Scientific Diagram

The dissociation constant of a week monobasic acid is 3.5 × 10^-8 . calculate its degree of dissociation in 0.05 M solution. ( 8.37 × 10^-4 )

equilibrium - How to calculate the dissociation constant of a weak acid from the titration with a strong base? - Chemistry Stack Exchange