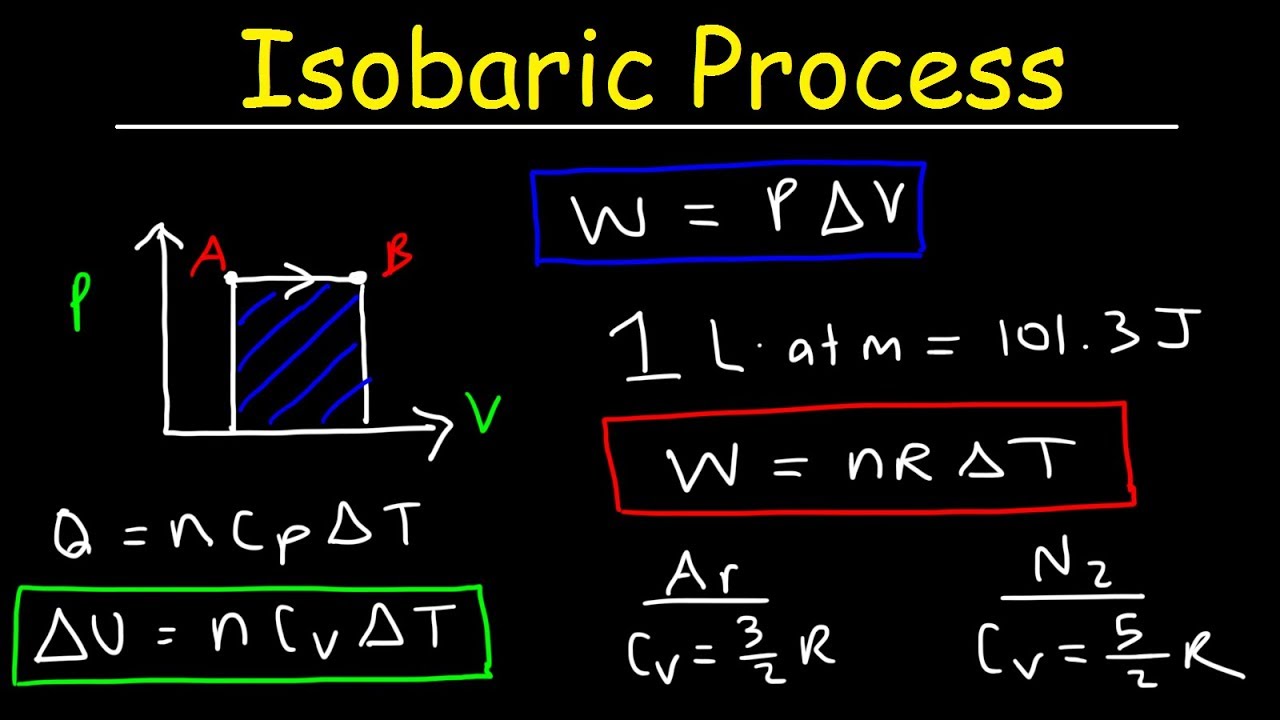
Isobaric Process Thermodynamics - Work & Heat Energy, Molar Heat Capacity, & Internal Energy - YouTube
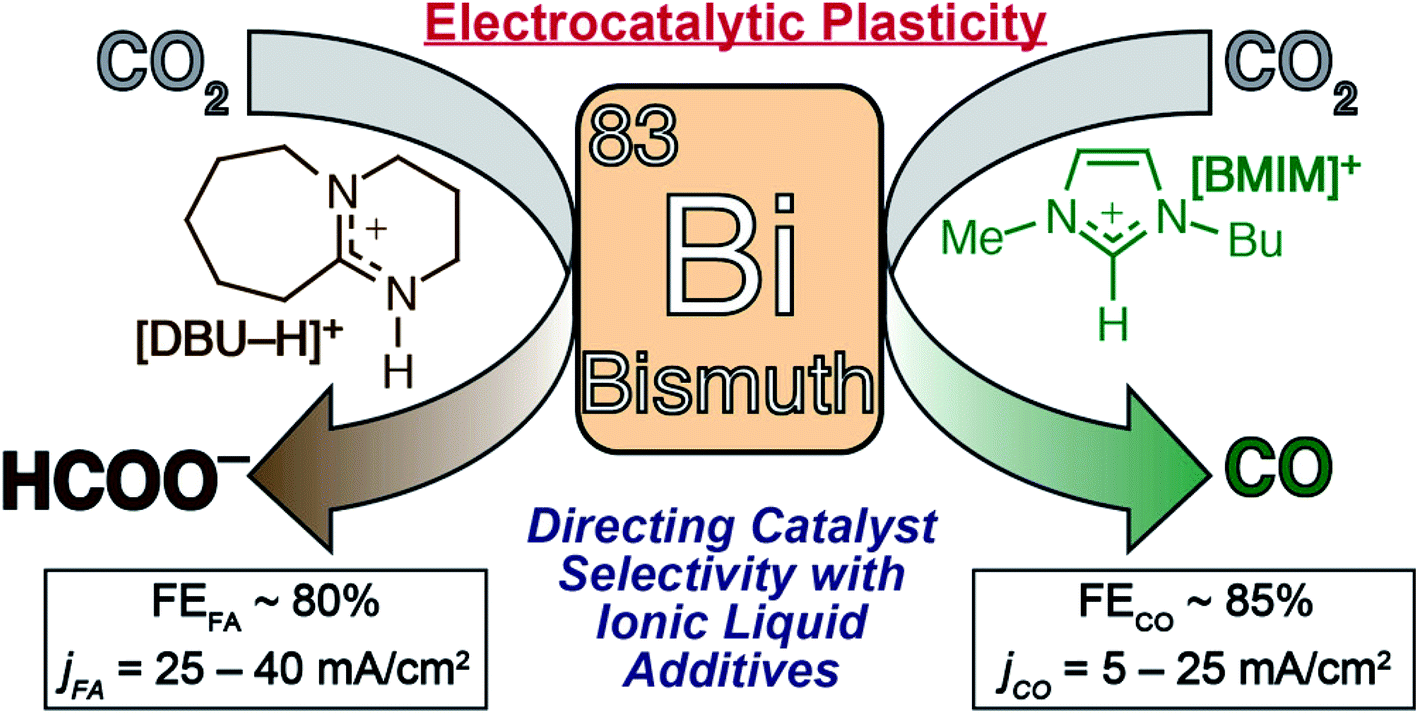
Electrochemical reduction of carbon dioxide (CO 2 ): bismuth-based electrocatalysts - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/D1TA01516H
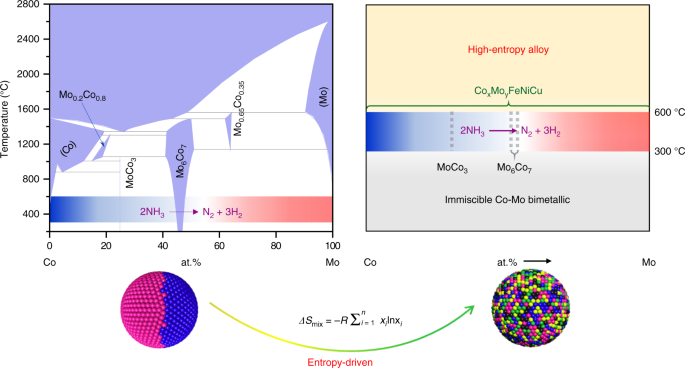
Highly efficient decomposition of ammonia using high-entropy alloy catalysts | Nature Communications

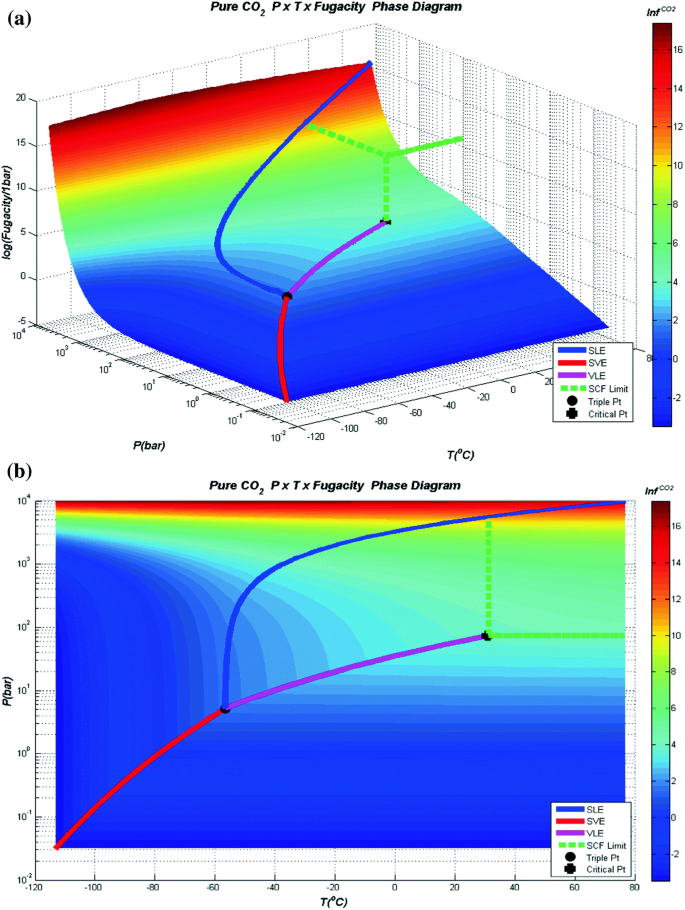
An Accurate and Efficient Look-up Table Equation of State for Two-Phase Compressible Flow Simulations of Carbon Dioxide | Industrial & Engineering Chemistry Research

CO2-Promoted Reactions: An Emerging Concept for the Synthesis of Fine Chemicals and Pharmaceuticals | ACS Catalysis

Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials - Han - Carbon Neutralization - Wiley Online Library

How many grams of carbon dioxide gas is dissolved in a 1 L bottle of carbonated water if the manufacturer uses a pressure of 2.4 atm in the bottling process at 25 ^

Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O | ACS Catalysis

Effects of Particle Diameter and Inlet Flow Rate on Gas–Solid Flow Patterns of Fluidized Bed | ACS Omega

Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks | Inorganic Chemistry

Calculate the entropy change when 2.8g of N2 gas expands isothermally and reversibly from an initial volume of 1L to final volume of 10L at 27^∘ C
DOT US Department of Transportation PHMSA Pipeline and Hazardous Materials Safety Administration OPS Office of Pipeline Safety

Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials - Han - Carbon Neutralization - Wiley Online Library

22 g of CO2 at 27^0 C is mixed in a closed container with 16 g of O2 at 37^0 C. It both gases are considered as ideal kinetic theory gases, then

Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks | Inorganic Chemistry