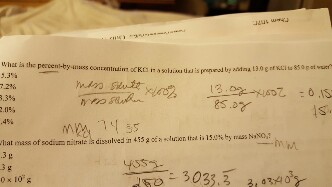The density of `3M` solution of `NaCl` is `1.25 g mL^(-1)`. The molality of the solution is... - YouTube

An aqueous solution contains 30% w/v of urea , density of solution is 1.2 g/ml. Calculate mass of water in 100ml solution.

Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors | Molecular Psychiatry

Utilization of Low-Concentration CO2 with Molecular Catalysts Assisted by CO2-Capturing Ability of Catalysts, Additives, or Reaction Media | Journal of the American Chemical Society
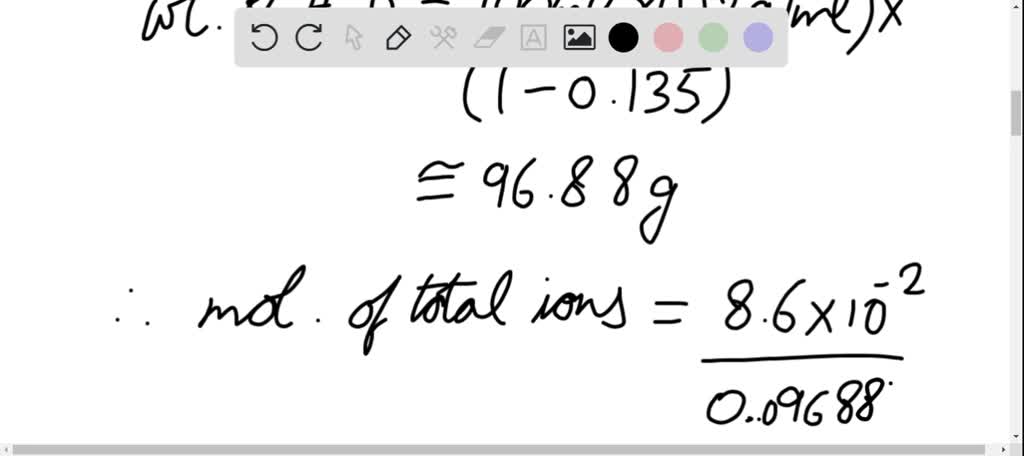
SOLVED:A 100.0 -mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g / mL . What would you add (solute or solvent) and what mass

Small molecule SWELL1 complex induction improves glycemic control and nonalcoholic fatty liver disease in murine Type 2 diabetes | Nature Communications

Calculate the molality of the KOH solution having density 1.5 g/mL, when the molarity of the same - Brainly.in

A solution is prepared by dissolving 4 g of NaOH to give 500 ml of it. Calculate the molality of the solution.
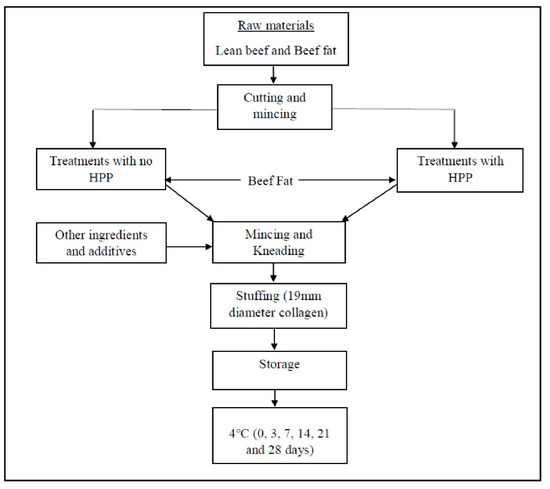
Processes | Free Full-Text | Effect of Partial Substitution of Sodium Chloride (NaCl) with Potassium Chloride (KCl) Coupled with High-Pressure Processing (HPP) on Physicochemical Properties and Volatile Compounds of Beef Sausage under

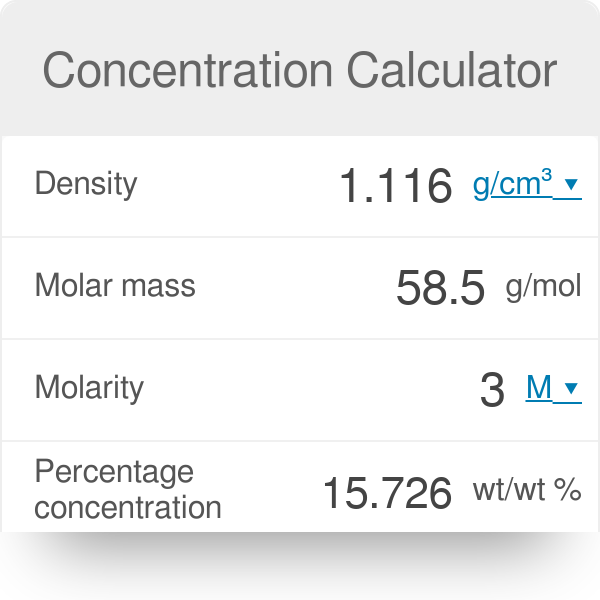
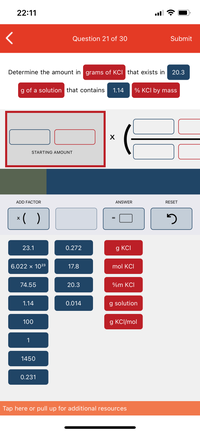
The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.
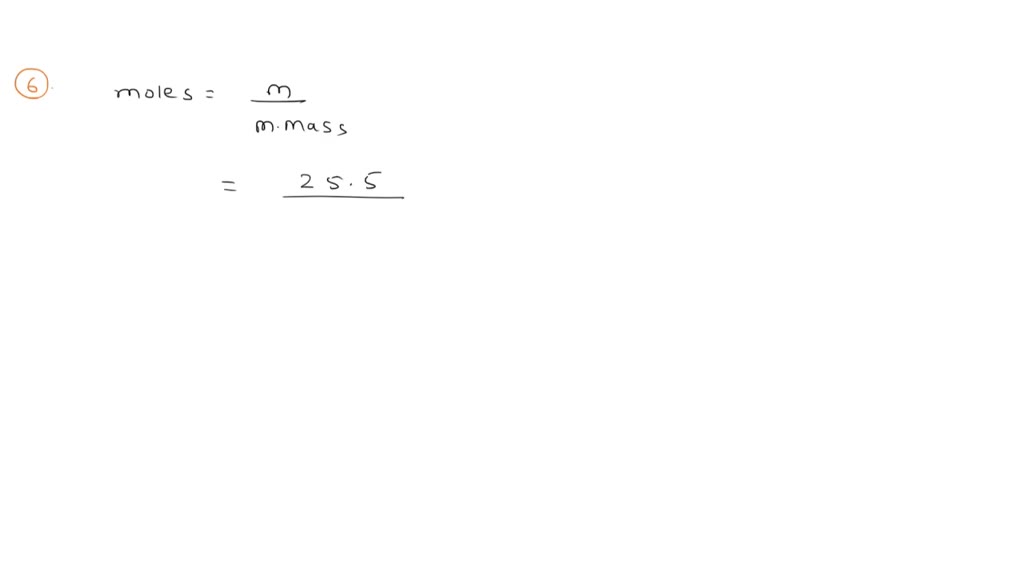
SOLVED: What is the Molar concentration of a solution with a volume 3.3 mL that contains 12 grams of ammonium sulfite? How many grams of copper (II) fluoride are needed to make

Cells in New Light: Ion Concentration, Voltage, and Pressure Gradients across a Hydrogel Membrane | ACS Omega

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.

A solution of KCl has a density of 1.69 g mL^(-1) and is 67% by weight. Find the denisty of the solution if it is diluted so that the percentage by weight